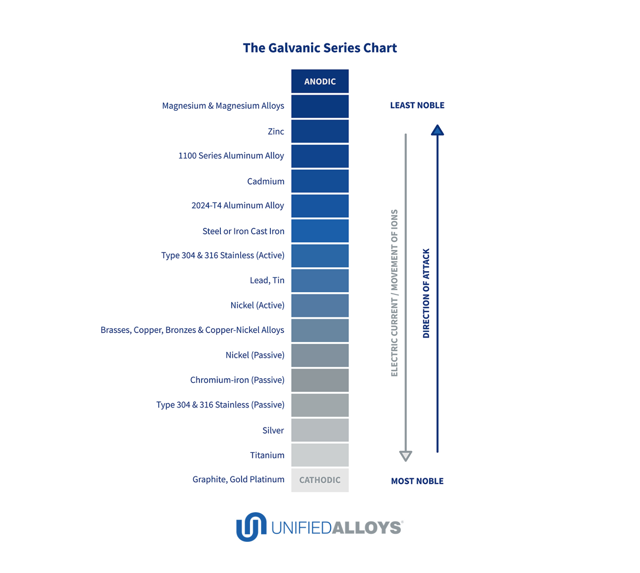
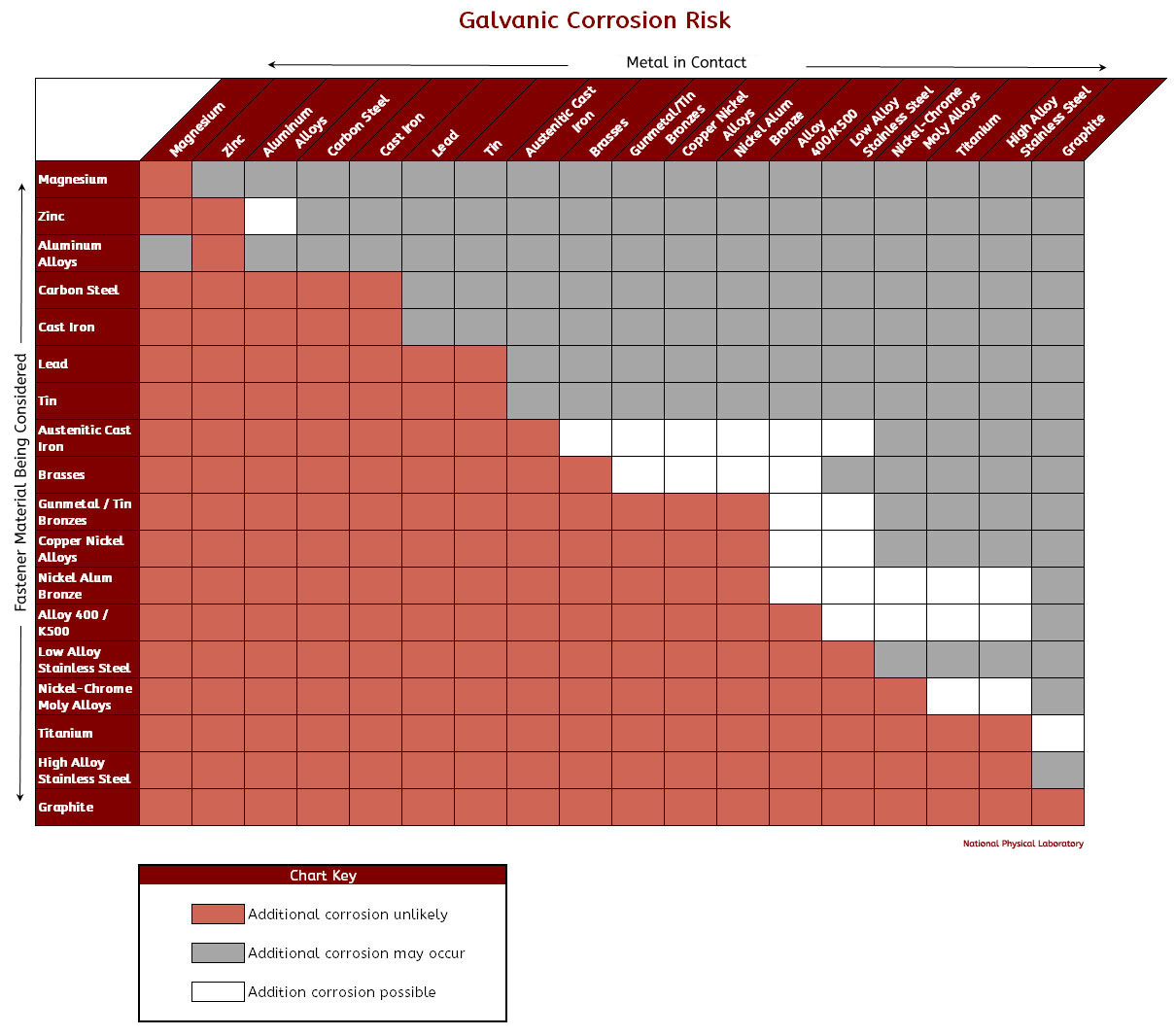
Galvanic Coupling Chart
Galvanic Coupling Chart - The following galvanic table lists metals in the order of their relative activity in seawater environment. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. Metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion. Galvanic series / galvanic table. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. In this article, we'll look at an example to illustrate the use of the galvanic table. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. Web there are two primary types of galvanic cells that cause corrosion: Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. In this article, we'll look at an example to illustrate the use of the galvanic table. ~ fe 2+ + 2e) and there are several possible cathodic reactions: Web figure 3a shows the galvanic corrosion of carbon steel bolts used to secure a stainless steel structural railing support on a bridge. The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Web often when design requires that dissimilar metals come in contact, the galvanic compatibility is managed by finishes and plating. The list begins with the more active (anodic) metal and proceeds down. Metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). Web there are two primary types of galvanic cells that cause corrosion: Hydrogen evolution (acids) 2h + + 2e ~ h2. Web in each solution, it is possible to establish a « galvanic series », i.e. Web the. This form of corrosion has the potential to attack junctions of metals, or regions where one construction Web often when design requires that dissimilar metals come in contact, the galvanic compatibility is managed by finishes and plating. Metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). The. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. This form of corrosion has the potential to attack junctions of metals, or regions where one construction In this article, we'll look at an example to illustrate the use of the galvanic table.. Web galvanic corrosion is a localised mechanism by which metals can be preferentially corroded. The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion. Web below is a galvanic reaction chart for dissimilar metals. The most active metals in the galvanic corrosion chart, like aluminum, zinc, or magnesium, are more likely. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web below is a galvanic reaction chart for dissimilar metals. In this article, we'll look at an example to illustrate the use of the galvanic table. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help. The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. So, for example, choosing zinc on zinc would have the lowest risk for. Web there are two primary types of galvanic cells that cause corrosion: Web below is a galvanic reaction chart for dissimilar metals. This form of corrosion has the potential to attack junctions of metals, or regions where one construction Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web by knowing the relationships of the metals. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. The finishing and plating selected facilitate the dissimilar materials being in contact and. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. In this article, we'll look at an example to illustrate the use of the galvanic table. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: The galvanic series indicates which dissimilar metal will tend to corrode (anode). Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Hydrogen evolution (acids) 2h + + 2e ~. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web the galvanic series compatibility of different metals can be assessed, relative to the potential for galvanic corrosion, with the use of charts depicting the galvanic (or electromotive force) series in different environments. We also provide other helpful methods for avoiding galvanic corrosion. Web by knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic corrosion. Web below is a galvanic reaction chart for dissimilar metals. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web there are two primary types of galvanic cells that cause corrosion: A classification of the different metals and alloys according to this measured potential (see chart below). The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. You can also learn more about overcoming potentially compatibility issues between metals. The list begins with the more active (anodic) metal and proceeds down. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. This form of corrosion has the potential to attack junctions of metals, or regions where one constructionGalvanic Corrosion Chart Metals
Galvanic Corrosion Chart
Galvanic Corrosion Cable Cleats CMP Products Limited
Galvanic Corrosion Chart Industrial Metal Service
Galvanic Corrosion A Guide for Architects (with a Galvanic Series Chart)
Galvanic Chart FINE METAL ROOF TECH
Galvanic Action Corrosion Prevention Architect's Blog
Stainless Steel Galvanic Corrosion Chart
Chemical Resistance Chart For Metals
Galvanic Corrosion Chart Metals
This Chart Is Designed To Assist In Broadly Assessing The Risk Of Galvanic Corrosion Associated With A Given Metal Coming Into Contact With Another Metal.
Web Figure 3A Shows The Galvanic Corrosion Of Carbon Steel Bolts Used To Secure A Stainless Steel Structural Railing Support On A Bridge.
Web Below, We Give A Brief Overview Of Galvanic Corrosion And Provide A Galvanic Corrosion Chart To Help Fabricators And Machinists Avoid Using The Wrong Metal Combinations.
Web Galvanic Corrosion Is A Localised Mechanism By Which Metals Can Be Preferentially Corroded.
Related Post: