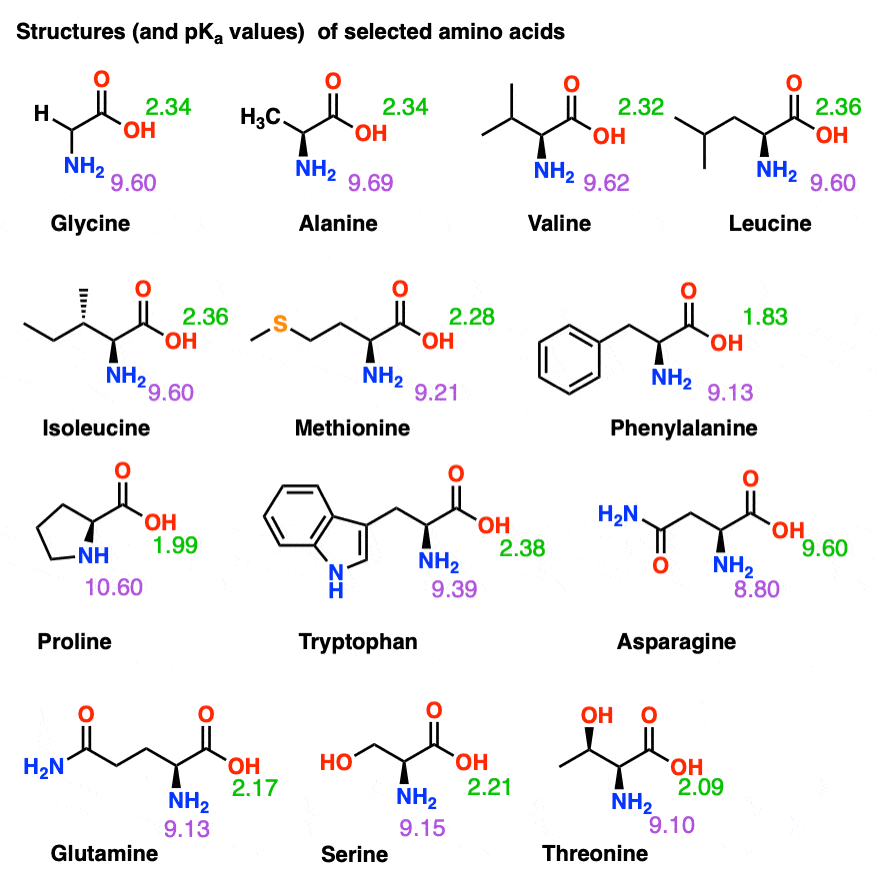
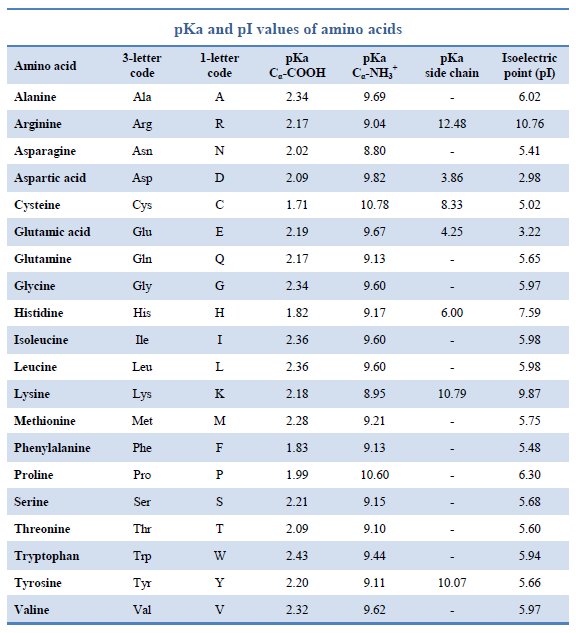
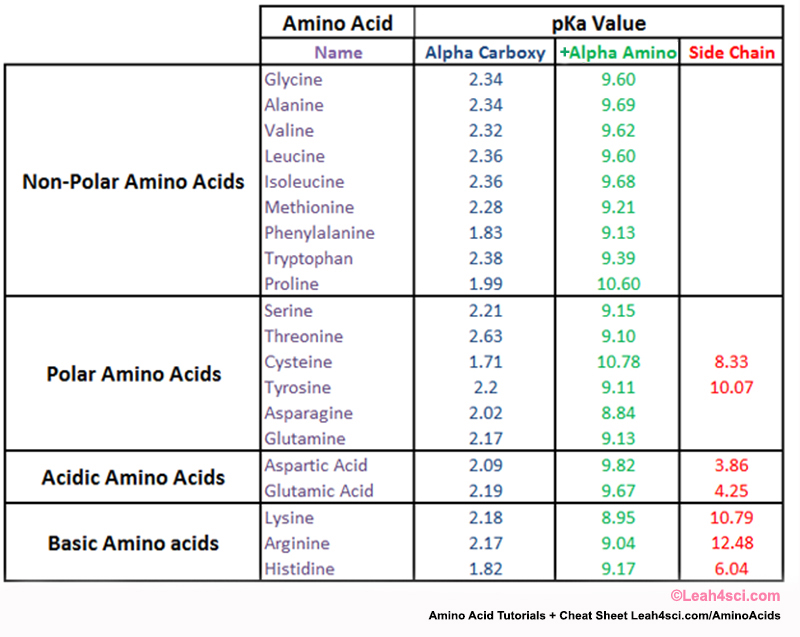
Amino Acid Chart With Pka
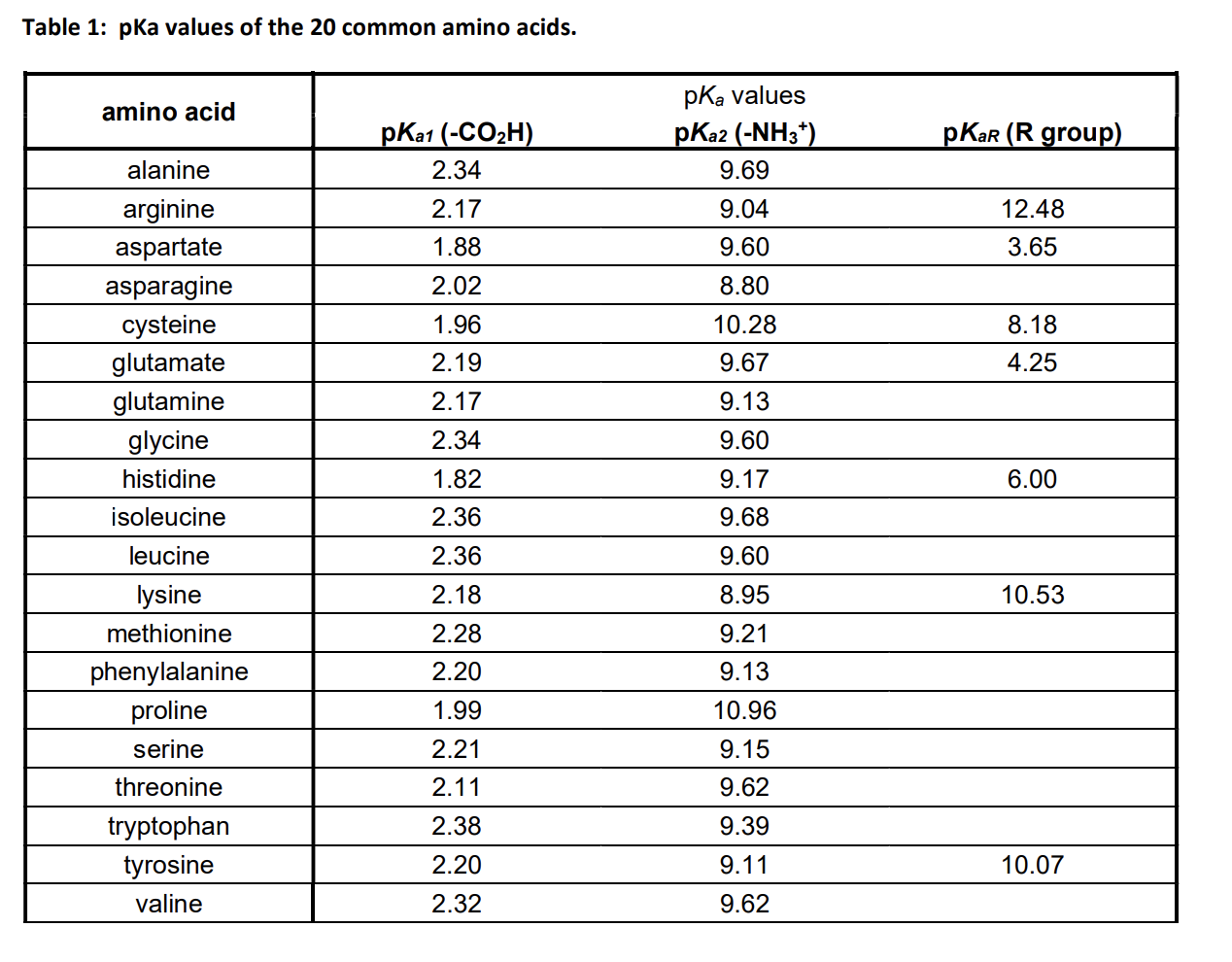
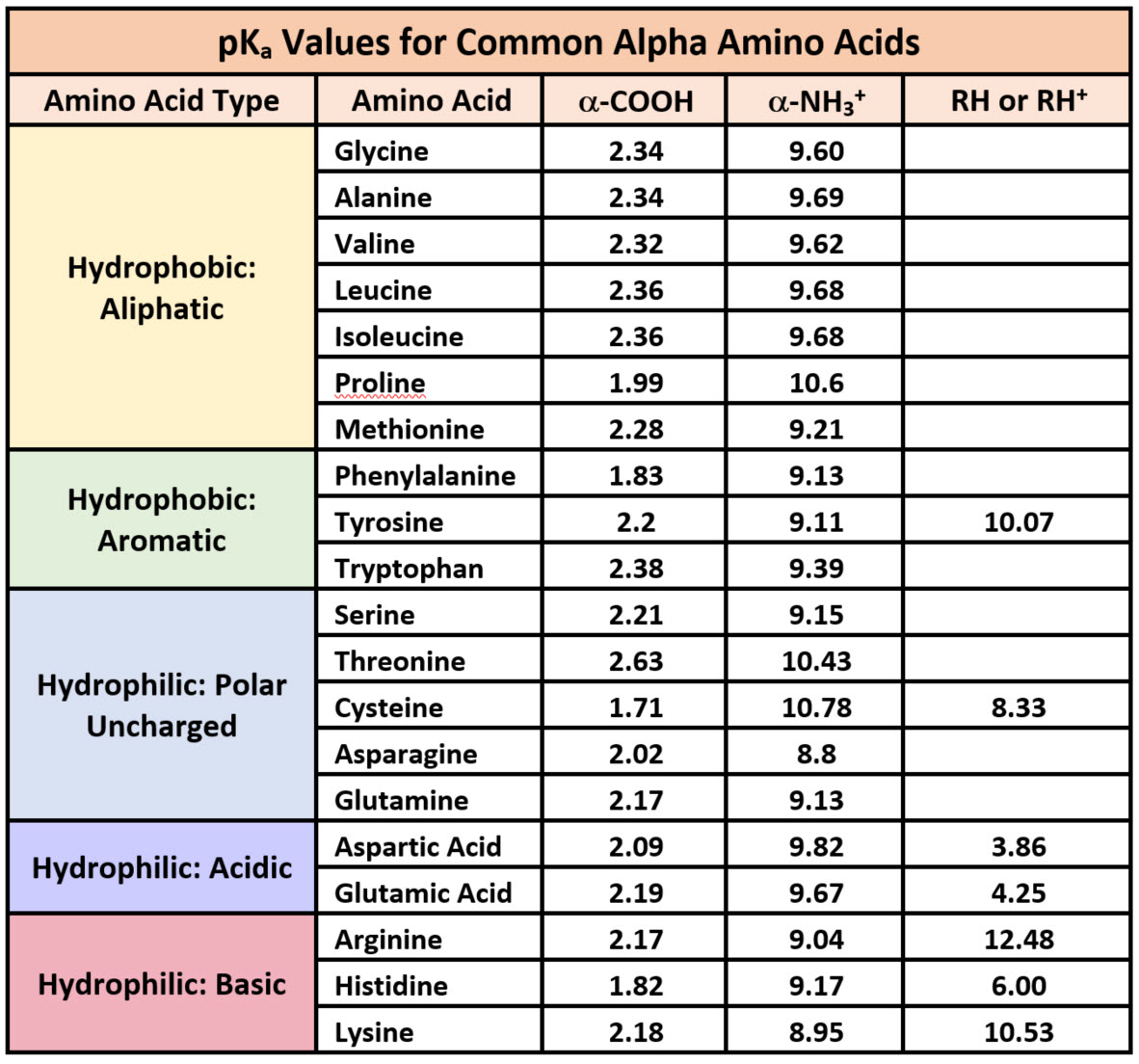
Amino Acid Chart With Pka - The isoelectric point, pi, is the ph at which negative and positive charges are balanced. Web the r group, which differs for each amino acid, will determine its structure, polarity and ph. Web most biochemistry courses will require you to know the following: The isoelectric points range from 5.5 to 6.2. For the four amino acids with either a strongly or weakly acidic side chain, pi is the average of the two lowest pk a values. Thus, amino acids with (chemically) similar side groups can be expected to function in similar ways, for example, during protein folding. Glycine alanine valine leucine isoleucine proline serine threonine cysteine methionine asparagine glutamine phenylalanine tyrosine tryptophan lysine arginine histidine aspartate glutamate 2.4 2.4 2.3 2.3 2.3 2.0 2.2 2.1 1.9 2.1 2.1 2.2 2.2 2.2 2.5 2.2 1.8 1.8 2.0 Web table of contents. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Amino acids are the building blocks of proteins. Web the r group for each of the amino acids will differ in structure, electrical charge, and polarity. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Web table of pk a and pi values. For example, the pi of the amino acid glycine is around 5.97, while the pi of lysine, with a basic side chain, is approximately 9.74. Web using a pka table. The chemical nature of this side chain determines the unique properties of each amino acid. How to use a pka table to determine relative acid strengths. Amino acid pka c pka n pka r pi; For the 13 amino acids with a neutral side chain, pi is the average of pk a1 and pk a2. The chemical nature of this side chain determines the unique properties of each amino acid. Summary of pkas of amino acids Web the r group, which differs for each amino acid, will determine its structure, polarity and ph. Web the r group for each of the amino acids will differ in structure, electrical charge, and polarity. Thus, amino acids with. Glycine alanine valine leucine isoleucine proline serine threonine cysteine methionine asparagine glutamine phenylalanine tyrosine tryptophan lysine arginine histidine aspartate glutamate 2.4 2.4 2.3 2.3 2.3 2.0 2.2 2.1 1.9 2.1 2.1 2.2 2.2 2.2 2.5 2.2 1.8 1.8 2.0 For the 13 amino acids with a neutral side chain, pi is the average of pk a1 and pk a2. Amino. Web karen steward, phd. Web for amino acids with only one ionizable acidic group (carboxyl group) and one ionizable basic group (amino group), the pi can be calculated as the average of the two pka values. Amino acid pka c pka n pka r pi; Web the r group, which differs for each amino acid, will determine its structure, polarity. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Summary of pkas of amino acids For the four amino acids with either a strongly or weakly acidic side chain, pi is the average of the two lowest pk a values. For the 13 amino acids with a neutral. Web table \(\pageindex{2}\) shows the standard pk a values for the amino acids and can be used to predict the ionization/charge status of amino acids and their resulting peptides/proteins. Refer to chart below to explore structures, properties and types for each of the 20 standard amino acids. Amino acids are the building blocks that form polypeptides and ultimately proteins. Web. Pi calculations for amino acids with basic side chains. Glycine alanine valine leucine isoleucine proline serine threonine cysteine methionine asparagine glutamine phenylalanine tyrosine tryptophan lysine arginine histidine aspartate glutamate 2.4 2.4 2.3 2.3 2.3 2.0 2.2 2.1 1.9 2.1 2.1 2.2 2.2 2.2 2.5 2.2 1.8 1.8 2.0 Register for free to listen to this article. That is a daunting. Want to join the conversation? At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Summary of pkas of amino acids The isoelectric point, pi, is the ph at which negative and positive charges are balanced. For example, let's consider alanine (ala) again. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Web all amino acids have the same basic structure, which is shown in figure 2.1. Web for amino acids with only one ionizable acidic group (carboxyl group) and one ionizable basic group (amino group), the pi can be calculated as the average of the two pka. Pi calculations for amino acids with acidic side chains. How to use a pka table to determine relative acid strengths. Web the r group for each of the amino acids will differ in structure, electrical charge, and polarity. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Calculating isoelectric point pi from pka values. The chemical nature of this side chain determines the unique properties of each amino acid. Pi calculations for amino acids with basic side chains. Web amino acid structure (video) | khan academy. They contain an amino group, carboxylic acid group, alpha carbon, and side chain. Web the r group for each of the amino acids will differ in structure, electrical. For the four amino acids with either a strongly or weakly acidic side chain, pi is the average of the two lowest pk a values. Glycine alanine valine leucine isoleucine proline serine threonine cysteine methionine asparagine glutamine phenylalanine tyrosine tryptophan lysine arginine histidine aspartate glutamate 2.4 2.4 2.3 2.3 2.3 2.0 2.2 2.1 1.9 2.1 2.1 2.2 2.2 2.2 2.5 2.2 1.8 1.8 2.0 Web table of contents. They contain an amino group, carboxylic acid group, alpha carbon, and side chain. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Calculating isoelectric point pi from pka values. Want to join the conversation? Web table of pk a and pi values. Amino acids are the building blocks of proteins. That is a daunting task for 20 amino acids. Web using a pka table. Web most biochemistry courses will require you to know the following: How to use a pka table to determine relative acid strengths. Web karen steward, phd. For example, the pi of the amino acid glycine is around 5.97, while the pi of lysine, with a basic side chain, is approximately 9.74. Pi calculations for amino acids with basic side chains.Amino Acid Chart With Pka
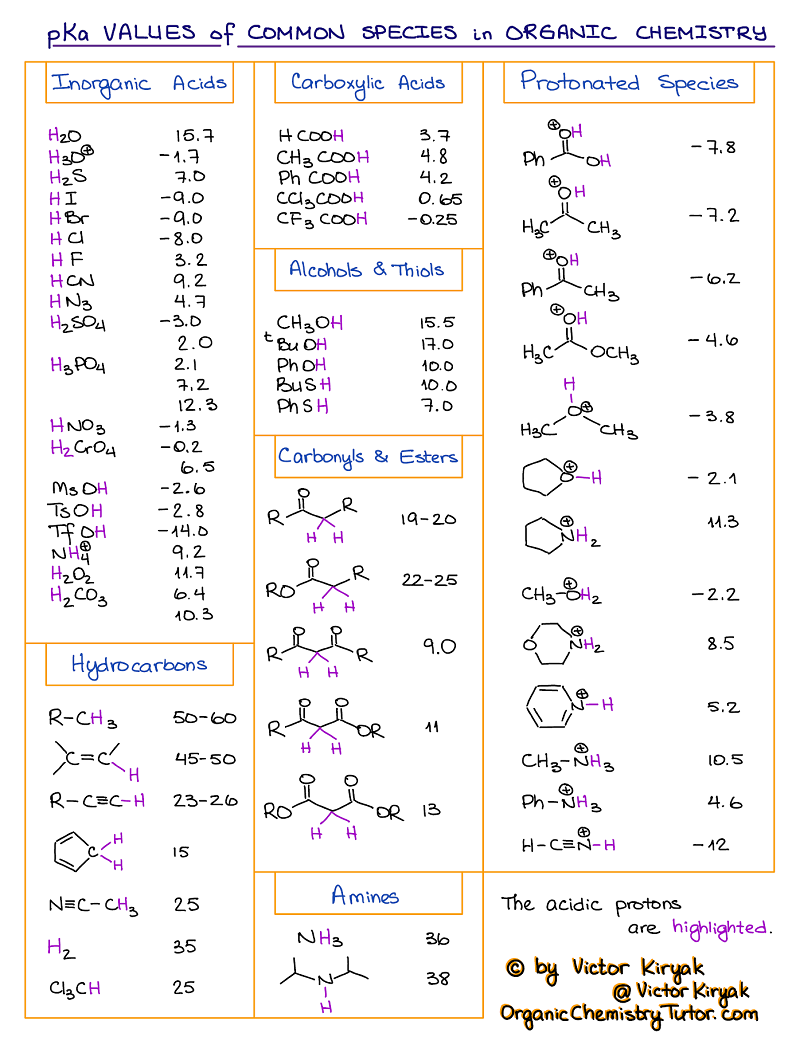
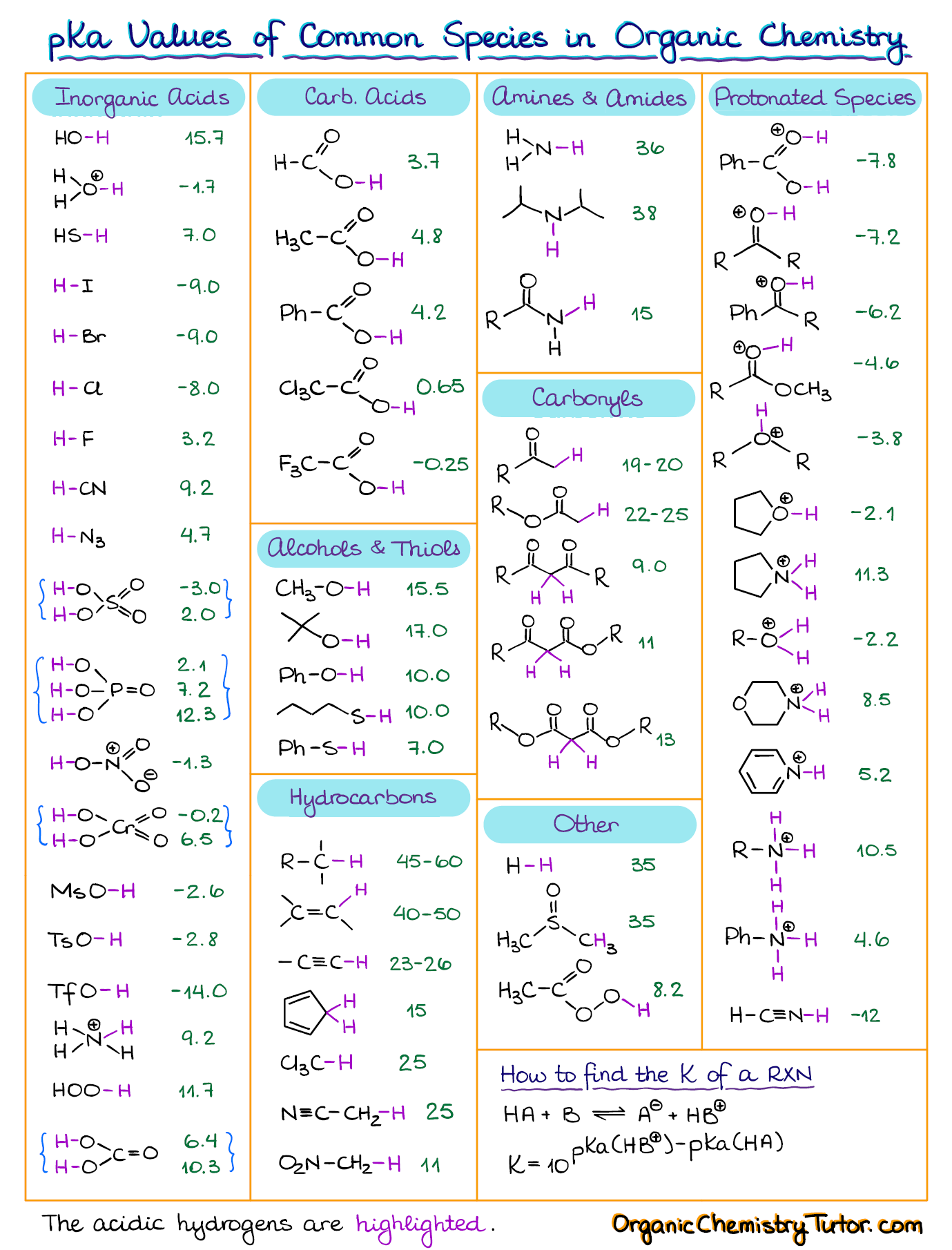
pKa Table and How to Use It — Organic Chemistry Tutor
Amino Acid Chart Pka
Amino Acid Pka Chart A Visual Reference of Charts Chart Master
Amino Acid Study Guide Structure and Function Albert.io
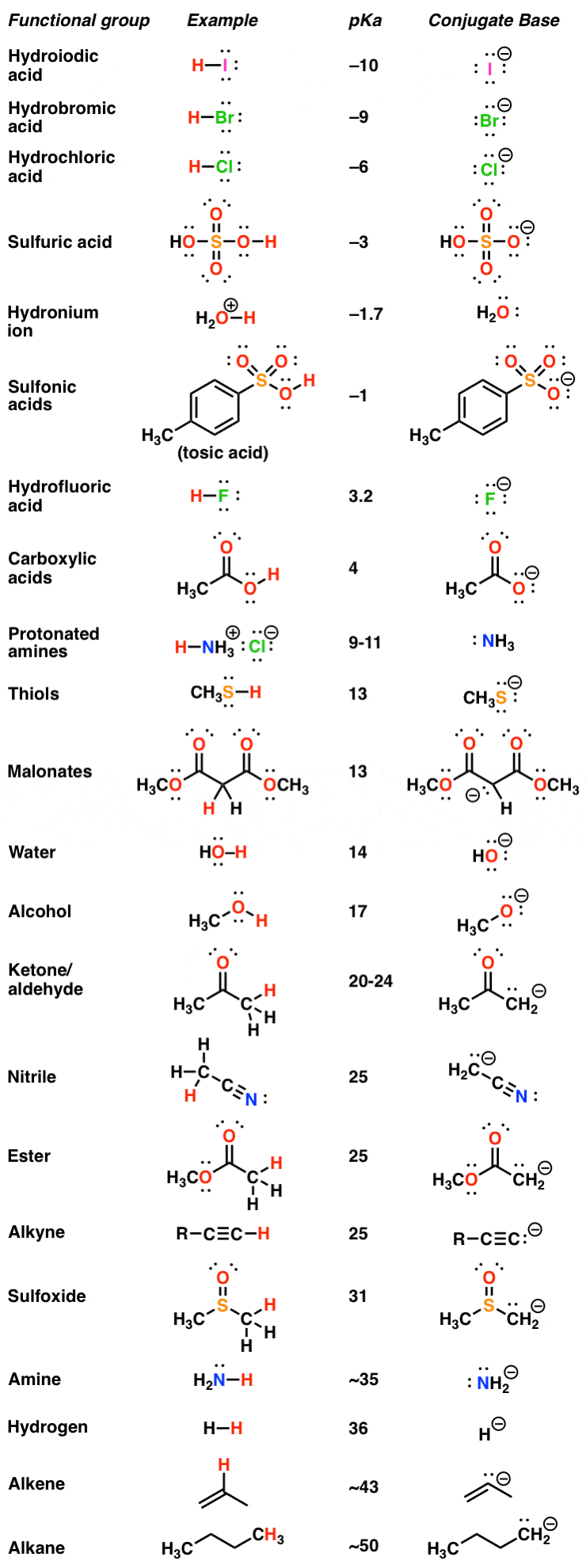
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic
Amino Acids And Pka Values
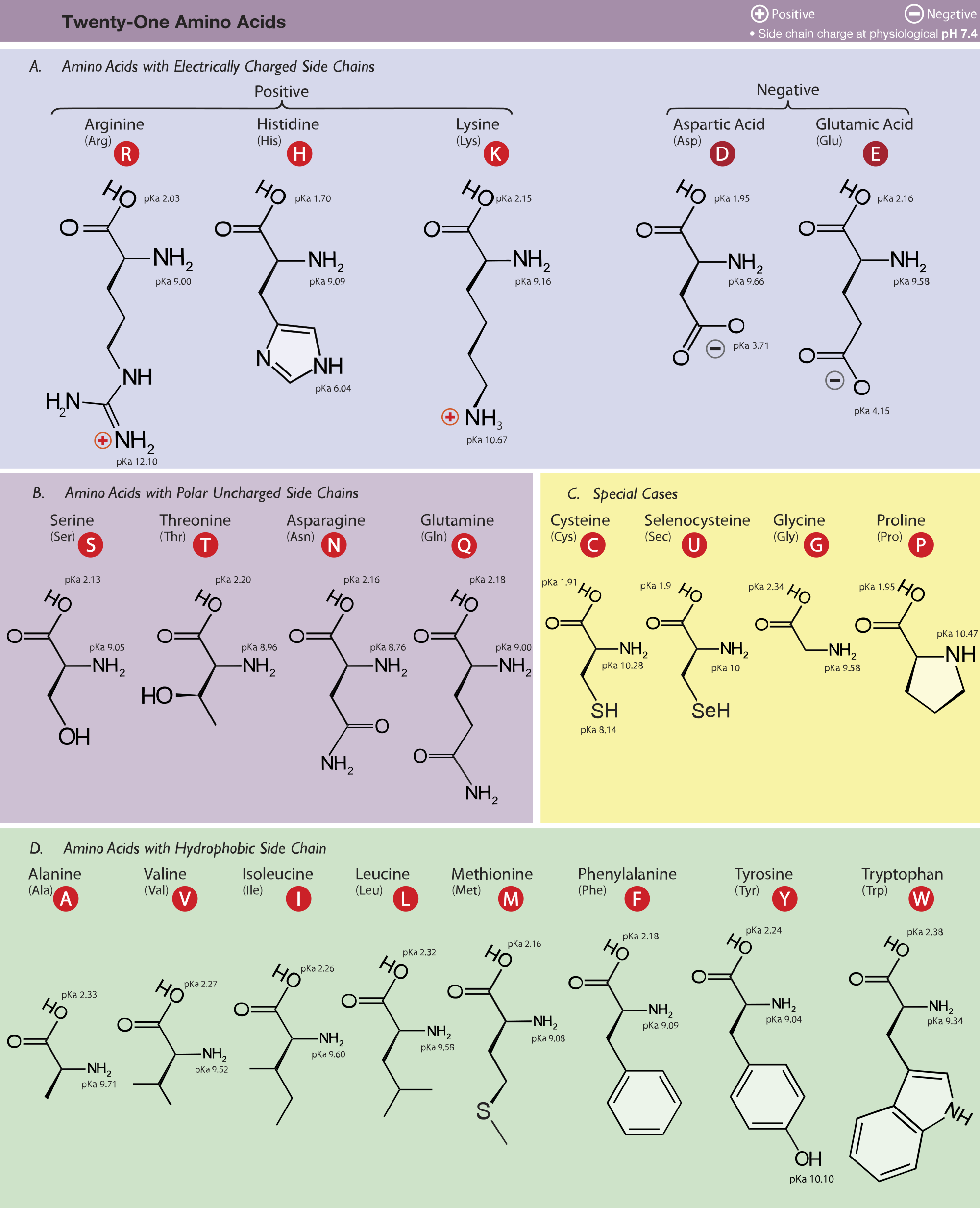
Amino acid properties
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial
3.1 Amino Acids and Peptides Biology LibreTexts
Web The R Group, Which Differs For Each Amino Acid, Will Determine Its Structure, Polarity And Ph.
Amino Acids Are The Building Blocks That Form Polypeptides And Ultimately Proteins.
Web Amino Acid Structure (Video) | Khan Academy.
Refer To The Charts And Structures Below To Explore Amino Acid Properties, Types, Applications, And Availability.
Related Post: